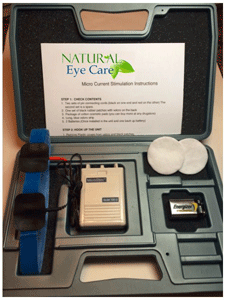
Microcurrent Stimulation 100ile Purchase Option
Price: $1250 per unit, or leasing option available)
Microcurrent Stimulation helps stimulate energy production (ATP) in the retina, improve circulation & reduce waste build-up.
MicroCurrent Stimulation (MCS) 100ile is an enhanced adaptation of a FDA approved therapy used by anesthesiologists, orthopedic surgeons, plastic surgeons and rehabilitative specialists to promote the healing of wounds and transplanted tissues as well as to treat pain.
Safety | Research | Warranty | Parts | Contraindications
Reviews
WHAT IS UNIQUE ABOUT THIS UNIT
Not only is this the only unit based on all research studies done to date, but it has a unique Regulator and Processor that uniquely adjusts the microcurrent based on each individual's retinal tissue acceptance of the microcurrent (1,000 times per second). The higher frequencies in the beginning of each 5-minute session helps relax the retinal tissue, then the lower frequencies stimulate the retina and ATP (energy) production within the retinal cells. The treatments also attracts adult stem cells to help stimulate possible cell regeneration.
HOW MCS WORKS
The theory is that MCS helps
- re-stimulate and energize dormant retinal cells (cells are like batteries -- when they run low in energy, they become sluggish and dormant),
- boost the cells' ability to rid themselves of waste products which interferes with the flow of energy, nutrients and communication,
- increase blood supply to the area stimulated. By increasing blood flow to the area, cells and tissues still living can get nourished and refreshed.
Although the exact mechanism of action of MCS has not yet been established scientifically, research suggests that microcurrent electrical stimulation device approximates the level of electrical activity present in a healthy eye, resulting in stimulating retinal activity and energizing dormant cells, as well as improving microvascular circulation, nerve conduction and velocity.
Microcurrent stimulation increases ATP (energy) synthesis in the retinal cells needed for membrane viability and waste management (a major concern for those with dry macular degeneration as excess waste not reabsorbed and eliminated results in waste accumulation called "drusen").
The treatment of patients with Macular Degeneration and Retinitis Pigmentosa entails the periodic administration of very precise amounts of tightly controlled electrical current through electrodes applied to the skin at specific areas around the eye. The electrical current is used to stimulate the retina as well as the diseased macula in order to help protect sight. The procedure is safe, noninvasive and painless and no side effects or adverse reactions have been observed.
There are several metabolic processes that are enhanced through the use of Microcurrent Stimulation. The first to boost the cells' ability to rid themselves of waste products. A cell with "stuck" waste products becomes a dead cell and interferes with cellular communication throughout the area where it is located. Cells need to take in nutrients and eliminate waste like all other living organisms. The energy supplied by Microcurrent Stimulation innervates cells to become vital and less sluggish.
The second way Microcurrent Stimulation works is by increasing blood supply to the area stimulated. By increasing blood flow to the area cells and tissues are nourished, refreshed and oxygenation is increased.
In general, the electrical current gently wakes up the cells from sleep and stimulates the healing process.
WHY WE OFFER THIS PARTICULAR UNIT:
This unit contains a microprocess to tightly control output. The units adjusts the current output over 1000 times a second, making sure that the current remains constant. Current going through tissue gives resistance so the current needs to remain constant to be effective. Less expensive units do not provide this necessary function and are not appropriate for eye health.
In addition this unit uses carrier waves which creates a more complex waveform and one that is capable of penetrating the body to the level where eye support is needed.
These are very safe machines. They are battery powered and designed so that they can’t generate enough current to damage tissue. They are shielded. The amount of current a unit can deliver is considered physiologic, meaning that the currents generated are currents on a level that the body would produce naturally.
This is the home unit version of the Microcurrent Stimulation (MCS 100 ile). The frequency settings were used in the studies described below. It is preset automatically to run through 4 frequencies in a preset 5-minute cycle before turning off. The four frequencies are: 292Hz for 30 seconds, 30Hz for 30 seconds, 9.1Hz for 2 minutes, then finally .3Hz for 2 minutes. Voltage is up to 22 and amps range 700 microAmps.
There are other microcurrent stimulation units available, but they have to be specifically calibrated both in frequency and timing to ensure proper gentle wave currents for your eyes. This unit works on alternating current. The output is DC with elements of AC introduced on each cycle per second.
Most of these units are actually TENS units designed more for wound healing and bone repair.
IS MICROCURRENT STIMULATION SAFE:
No side effects or adverse outcomes related to this treatment have been seen so far. No increase in the conversion to the wet form of ARMD has been seen to those who have been treated.
In a consensus statement by the NIH reports that, "One of the advantages...is that the incidence of adverse affects is substantially lower than that of many drugs or other accepted medical procedures used for the same conditions.
RESEARCH
3 month pilot, 17 patients, Laurie Chaikin
(AMD)
- Dry macular degeneration, once a week treatment, 52% showed improvement
- Wet macular degeneration, improvement but not statistically significant
- Only treated once a week
- Published 2015, see details
2 year study, 114 patients, Grace Halloran, PhD
- 18 patients with AMD - 16 improved.
- 78 had RP - 62 improved
- 18 patients had various retinopathies, 16 improved.
- 14 remained unchanged (stable)
- 2 continued to lose vision, although only slightly.
- Published 1997, see details
10 year clinical study, 400 eyes with AMD, Drs. Jarding and Michael
- 78% of the eyes showed from 1-9 lines of improvement in reading of the visual acuity chart.
- Over 50% improved from 2-9 lines.
- In the study, 2 patients suffered from retinal vein occlusion and swelling of the macula. Both had dramatic improvement in vision.
- Published 1993, see details
65 eyes, 36 sessions, Wallace
- Patient received MCS of 200 micro-amps for 20 minutes, 36 sessions
- 54% improved 1-4 lines of acuity
- 31% improved 2 to 4 lines acuity
- Kinetic visual field expanded by 85% horizontally for all patients
- Kinetic visual field expanded by 70% vertically for all patients
- Field improvements imply implications for Retinitis Pigmentosa
- Published 1997, see details
Review, 120 patients, Damon Miller, MD
Stargardt's Disease, Retinitis Pigmentosa and other degenerative retinal diseases
- That of the 120 patients treated, 83% showed improvement of greater than or equal to 2 lines of visual acuity in one or both eyes.
- 1997, see details
2 year trial, 120 patients, Macular Degeneration Foundation
- 68% improvement over pre-treatment vision for patients with the dry macular degeneration.
- 58% improvement over pre-treatment vision for those with the wet form of macular degeneration.
- Among certain subsets of patients with dry disease the results were even more dramatic, with a third gaining 100 percent or more improvement and a sixth gaining 150 percent or more improvement.
Patients were able to sustain these vision improvements over time by periodic self-administration of booster treatments and many report that this therapy has made a remarkable difference to their lives.
18 pilot month study, Dr. Ed Paul
(AMD, Stargardt's, Retinitis Pigmentosa)
- 68% showed an increase in vision function and visual acuity.
- Twenty-six out of 36 eyes with AMD showed an improvement in visual acuity. The acuity measured by Snellen, improved on average 2-3 lines.
- 2002, see details
Read more: How Microcurrent Stimulation Could Help Eye Diseases
FDA POSITION
MCS therapy is considered an "off label" use of an approved medical device. Off label usage of FDA approved devices and drugs are commonly practiced by doctors without interference from the FDA and allows doctors to practice in a manner they feel most beneficial to their patients.
DIAGNOSIS:
When ordering, please let us know your specific eye diagnosis.
CONTRAINDICATIONS:
MCS is contraindicated for those with pacemakers, or who are pregnant. If you have a neurological disorder such as epilepsy, please discuss with your doctor first before using MCS.How it Works Studies Safety Studies Pricing Abstracts FDA Note Directions Usage
DISCLAIMER:
The data presented here has not been reviewed by the FDA, nor has it been peer reviewed. The microcurrent devices used are approved by the FDA for the treatment of pain, but they have not been approved for other uses. The use of a device for an off-label use by a physician is legal. The use of microcurrent stimulation discussed here is only one part of a comprehensive program for supporting visual health, and should not replace any treatment prescribed by your physician.
WARRANTY
There is a full 1 year replacement warranty on any defective unit (based on normal wear and tear).
PARTS
The timer uses standard AAA batteries, the microstim unit uses a standard 9 volt battery, and you will be able to find replacement pads at your local drugstore (or we can order them for you.
| Free 1st Class Shipping | Free 1st Class Shipping |
|---|